Genome-wide sequencing-based identification of methylation quantitative trait loci and their role in schizophrenia risk
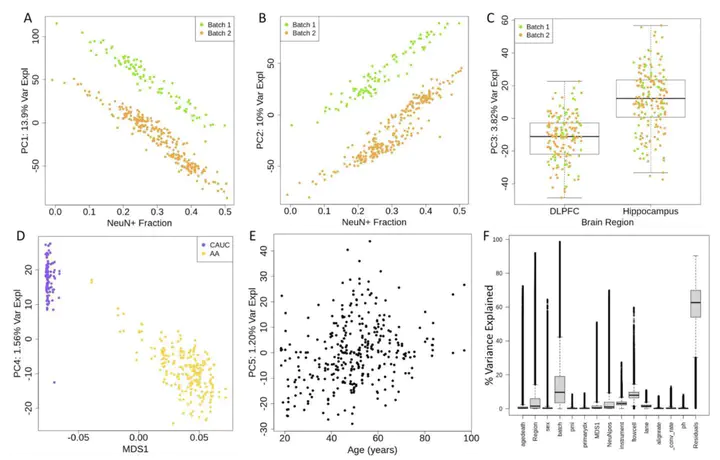 Image credit: bioRxiv
Image credit: bioRxiv
Abstract
DNA methylation (DNAm) is an epigenetic regulator of gene expression and a hallmark of gene-environment interaction. Using whole-genome bisulfite sequencing, we have surveyed DNAm in 344 samples of human postmortem brain tissue from neurotypical subjects and individuals with schizophrenia. We identify genetic influence on local methylation levels throughout the genome, both at CpG sites and CpH sites, with 86% of SNPs and 55% of CpGs being part of methylation quantitative trait loci (meQTLs). These associations can further be clustered into regions that are differentially methylated by a given SNP, highlighting the genes and regions with which these loci are epigenetically associated. These findings can be used to better characterize schizophrenia GWAS-identified variants as epigenetic risk variants. Regions differentially methylated by schizophrenia risk-SNPs explain much of the heritability associated with risk loci, despite covering only a fraction of the genomic space. We provide a comprehensive, single base resolution view of association between genetic variation and genomic methylation, and implicate schizophrenia GWAS-associated variants as influencing the epigenetic plasticity of the brain.
My latest paper is out in @NatureComms! We establish a single-base resolution view of the relationship between genetic variation and DNA methylation in the brain, and show how this can be used to better understand GWAS findings for schizophreniahttps://t.co/8juZlOjyAc
— Kira PM, PhD (@Kira_P_M) September 2, 2021
Excited to share the most comprehensive analysis to date of the associations between genetic variation and DNA methylation in the human brain! meQTLs are extensive throughout the genome and can likely be used to further understand GWAS risk variants:https://t.co/HGVUhtxPsi
— Kira PM, PhD (@Kira_P_M) September 25, 2020