Comprehensive assessment of multiple biases in small RNA sequencing reveals significant differences in the performance of widely used methods
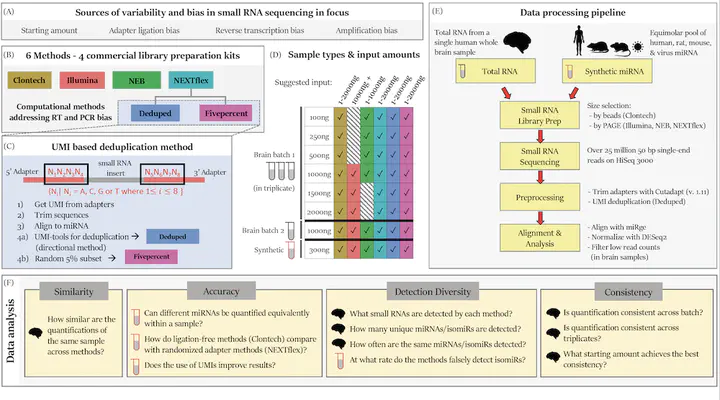 Image credit: bioRxiv
Image credit: bioRxiv
Abstract
Background: RNA sequencing offers advantages over other quantification methods for microRNA (miRNA), yet numerous biases make reliable quantification challenging. Previous evaluations of these biases have focused on adapter ligation bias with limited evaluation of reverse transcription bias or amplification bias. Furthermore, evaluations of the quantification of isomiRs (miRNA isoforms) or the influence of starting amount on performance have been very limited. No study had yet evaluated the quantification of isomiRs of altered length or compared the consistency of results derived from multiple moderate starting inputs. We therefore evaluated quantifications of miRNA and isomiRs using four library preparation kits, with various starting amounts, as well as quantifications following removal of duplicate reads using unique molecular identifiers (UMIs) to mitigate reverse transcription and amplification biases. Results: All methods resulted in false isomiR detection; however, the adapter-free method tested was especially prone to false isomiR detection. We demonstrate that using UMIs improves accuracy and we provide a guide for input amounts to improve consistency. Conclusions: Our data show differences and limitations of current methods, thus raising concerns about the validity of quantification of miRNA and isomiRs across studies. We advocate for the use of UMIs to improve accuracy and reliability of miRNA quantifications.
Interested in #smallRNA? Check out @LieberInstitute's new publication @BMC_series #BMCGenomics! 🎉 We found major differences in commonly used #rnaSeq #miRNAseq methods for #miRNA and #isomiR quantifications. https://t.co/rGTuCAnsOO pic.twitter.com/L9IEnMvGTJ
— mirnas22 (@mirnas22) July 2, 2019